Warming up with brown fat.
The paper: Gerhart-Hines, Z. et al. (2013) The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. doi:10.1038/nature12642
Subject areas: Physiology, Cell Biology
Vocabulary:
Zeitgeber Time (ZT) – ZT is a convention used in chronobiology to standardize time effects on biological processes. In the experiments described here, the mice are acclimated to a 24 hour light-dark cycle: lights on at 07:00, off at 19:00 normal time, corresponding to ZT 00:00 to 12:00. The term comes from German chronobiologist Jurgen Aschoff. Zeit means time in German; geber means giver.
exogenous – caused by or derived from factors outside of a system or organism. opposite of endogenous, which in biology usually means something generated by an organism/cell/tissue itself.
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biology, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
Most of us have been taught since we were little kids that mammals are warm-blooded animals and that we humans have a normal body temperature of about 37 ºC. Of course, at that age, we don’t really think much beyond that. The fact is, our body temperature actually changes throughout the day for a variety of reasons, and there is some very interesting control system biology going on to maintain body temperature in spite of large variations in the ambient temperature.
This paper explores some of the biological mechanisms at the intersection of circadian body temperature variations and body temperature adaptation to environmental changes. Circadian clocks are manifested by a network of interconnected transcription factors and the numerous effectors under their control managing to produce a 24-hour oscillation of many cellular and organismic functions. Circadian changes in body temperature are well documented: the core temperature is warmest while awake and coldest when askeep.
What they knew
Brown adipose tissue (BAT) is an important thermogenic (heat-producing) tissue in mammals, and that function is characterized by high glucose uptake (lots of fuel), oxidative capacity (“burning” the fuel), and mitochondrial uncoupling (using the released energy as heat instead of powering ATP formation). Rev-erbα is a circadian transcriptional repressor (prevents expression of a particular gene or set of genes) that can regulate glucose and lipid metabolism in white adipose and other tissues, but its role, if any, in brown adipose tissue is unknown.
What they did
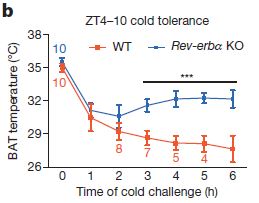 The first step was simply to see if Rev-erbα plays any role in temperature regulation. To do this, the researchers compared the response of wild-type(wt) and transgenic Rev-erbα knockout(ko) mice when challenged with acute cold, lowering the temperature of their environment from 29 ºC (84 ºF) to 4 ºC (39 ºF) in thirty minutes. The figure at right shows that although both sets of mice experience a core body temperature drop to ~30 C within an hour, the core temp of the mutant mice then holds steady, while the wild-type mice experience further body temperature drop, which is reflected in the survival of the mice (by 3 hours, all rev-erbα knockouts were alive, while half of the wild-type mice had died).
The first step was simply to see if Rev-erbα plays any role in temperature regulation. To do this, the researchers compared the response of wild-type(wt) and transgenic Rev-erbα knockout(ko) mice when challenged with acute cold, lowering the temperature of their environment from 29 ºC (84 ºF) to 4 ºC (39 ºF) in thirty minutes. The figure at right shows that although both sets of mice experience a core body temperature drop to ~30 C within an hour, the core temp of the mutant mice then holds steady, while the wild-type mice experience further body temperature drop, which is reflected in the survival of the mice (by 3 hours, all rev-erbα knockouts were alive, while half of the wild-type mice had died).
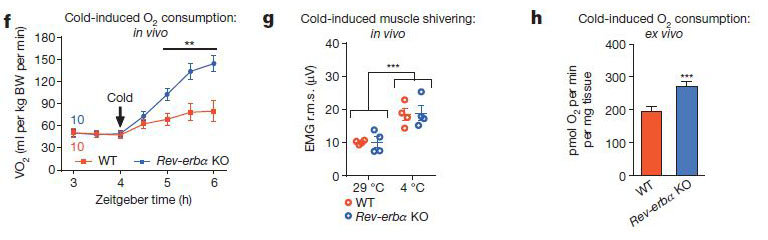
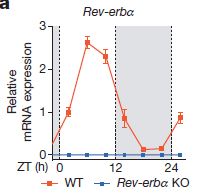 The interesting thing is that this difference only occurs when the experiment is run from Zeitgeber time (ZT, see vocabulary section above) 4:00-10:00. When the same experiment is run from ZT16-22, both wt and ko mice maintain a body temperature around 32 C, and there is 100% survival. This happens to coincide with a time-dependent drop in expression of Rev-erbα in the wild-type mice (figure at left). The question is then, what does Rev-erbα do? One clue could be in the oxygen consumption data: in the ZT 04:00-10:00 experiment, oxygen consumption ramped up strongly in the Rev-erbα knockouts compared to wild-type (f, below). However, the oxygen is probably not being used by shivering muscles to generate heat (g). When the brown fat (BAT) was isolated from cold-treated animals though, there was a significant increase in oxygen consumption by the Rev-erbα knockouts.
The interesting thing is that this difference only occurs when the experiment is run from Zeitgeber time (ZT, see vocabulary section above) 4:00-10:00. When the same experiment is run from ZT16-22, both wt and ko mice maintain a body temperature around 32 C, and there is 100% survival. This happens to coincide with a time-dependent drop in expression of Rev-erbα in the wild-type mice (figure at left). The question is then, what does Rev-erbα do? One clue could be in the oxygen consumption data: in the ZT 04:00-10:00 experiment, oxygen consumption ramped up strongly in the Rev-erbα knockouts compared to wild-type (f, below). However, the oxygen is probably not being used by shivering muscles to generate heat (g). When the brown fat (BAT) was isolated from cold-treated animals though, there was a significant increase in oxygen consumption by the Rev-erbα knockouts.
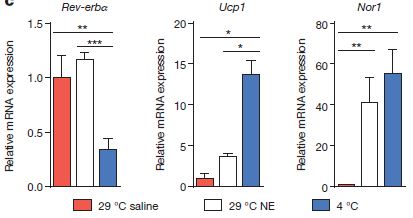
We already saw that there is a normal rapid drop in Rev-erbα expression every night in wt animals. Gerhart-Hines and his colleagues also noted that cold (4 ºC, blue bars, below) induced a strong drop in Rev-erba (left graph) in brown adipose tissue, compared to either normal temperature (29 ºC, red bars) or normal temperature with noradrenaline injection (white bars). They investigated the effect of noradrenaline because of a longstanding assumption that BAT thermogenesis is caused by release of the hormone noradrenaline, and activation of adrenergic signaling inside the BAT cells. There is a clear cold-induced increase in a nuclear receptor in the adrenergic signaling pathway (Nor1, right graph). There is also a cold-induced increase in Ucp1 (middle graph), which is an uncoupling protein, which as mentioned above, switches mitochondria from generating ATP to generating heat.
In fact, Ucp1 levels rise very quickly as Rev-erbα levels fall, suggesting that Rev-erba is a repressor of Ucp1. This is supported by the observation that even before cold-challenge, there is higher Ucp1 in the Rev-erba knockouts than in wild-type mice. In fact, when exogenous Rev-erbα is introduced to Rev-erbα knockouts, Ucp1 is reduced to wild-type levels (right).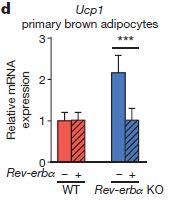
It seems clear that Rev-erbα is shut down in response to cold so that brown fat tissue can start thermogenesis. It is not so clear why there is a circadian pattern to Rev-erbα expression. One clue to that may be found in the an examination of core temperature in wt and Rev-erbα ko mice. The normal night-time drop in body temperature (at normal ambient temperatures) is abolished in Rev-erbα ko mice.
What it means
Brown adipose tissue likely evolved to allow mammals to survive a wide range of environmental temperatures. However, thermogenesis is extremely expensive to run, energetically (i.e. food) speaking. Therefore, a robust control mechanism likely evolved to balance the occasional need for thermogenesis and need to use food more efficiently in generating energy for the cells. The circadian rhythm of Rev-erbα is useful because at night when it is low, the body is able to automatically respond to sudden unexpected changes in environmental temperature without the animal needing to be awake to react. It appears that Rev-erbα is a temperature-dependent repressor of Ucp1, which controls thermogenesis in brown adipose tissue.

No comments
Be the first one to leave a comment.