Keep moving. It prevents your muscles from shriveling.
The paper: Yogev, O., et al. (2013) eIF4EBP3L Acts as a Gatekeeper of TORC1 In Activity-Dependent Muscle Growth by Specifically Regulating Mef2ca Translational Initiation. PLoS Biology 11(10): e1001679. doi:10.1371/journal.pbio.1001679
Subject areas: Physiology, Cell Biology, Developmental Biology
Vocabulary:
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biology, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
You probably looked at the title of today’s paper and thought, “What in the world is this about?” It is actually more interesting than you might guess. The key is in the middle of the title: activity-dependent muscle growth. Many of you probably think of that as building up your muscle like an athlete, which is certainly important. But it is also a serious health issue in the elderly or those who are physically unable to move easily. Learning more about the molecular basis of activity-dependent muscle growth could help to maximize the benefit of rehabilitative movement in inactive patients.
First, a basic cell biology review.
Any given cell in the body of an animal only uses some of the genes encoded in its DNA at a given time. Many genes are never used at all. However, when the cell does need to activate a gene and make a new protein product, it starts with the process of transcription. This means copying the information on the DNA, which must stay protected in the nucleus, onto a strand of RNA. The RNA can then be moved out of the nucleus to the cytoplasm, where the information can then translated into a protein. However, just because the RNA is out in the cytoplasm does not mean it is used immediately. One way that a cell can control the amount of a protein, but still be able to make it quickly, is to make the RNA transcripts, but not use them until needed. In order for translation of an RNA into protein to start, several proteins must come together at the start of the RNA. Conversely, to hold the RNA in the cytoplasm but not allow it to be translated, either the initiator proteins or the RNA must be blocked. eIF4EEBP3L is a protein that binds onto eIF4E (eukaryotic Initiation Factor 4E), preventing it from initiating translation.
What they did.
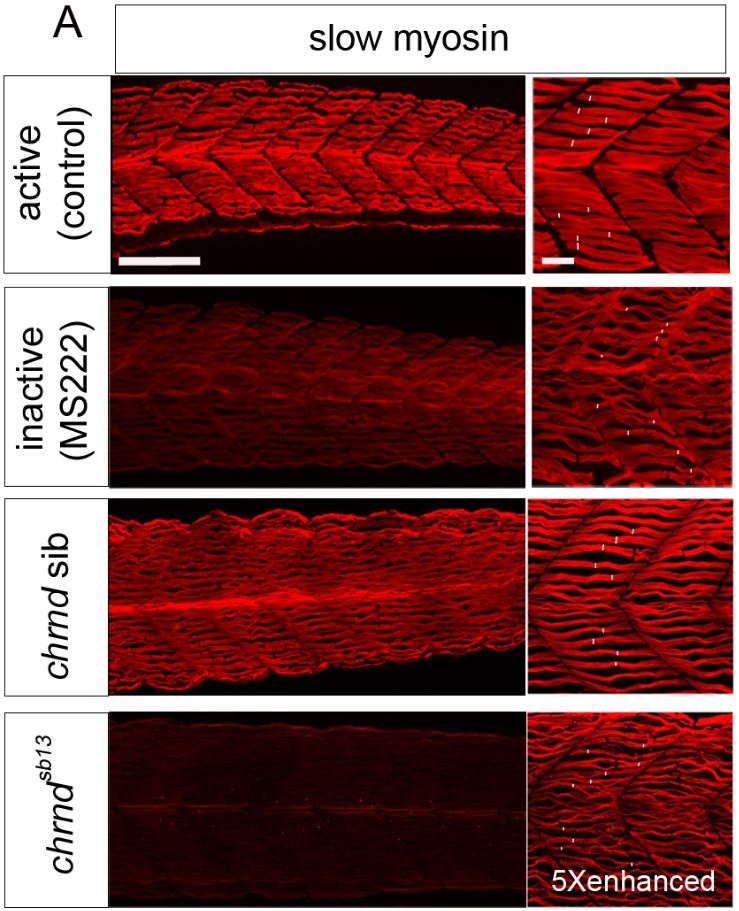
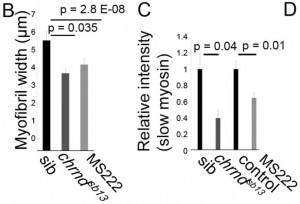
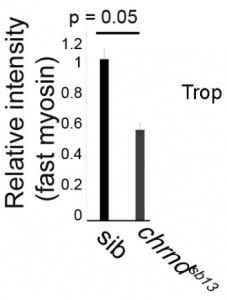
Muscle fibers can (and do) increase and decrease in size through the life of an animal, and this change is activity-dependent. In the system this study used, the developing zebrafish, muscle activity can be blocked either with drugs (tricaine mesylate, MS222) or using a genetic mutant that has a mutant acetylcholine receptor (chrnd), so the muscles cannot respond to the signals sent by the nerves. In figure (A) below, the control fish embryo has very well-defined bundling, and the slow myosin staining is very robust. In contrast, the MS222-treated myofibers and myofibrils from chrnd embryos both showed decreased slow myosin staining and bundling. There is also a decrease in fast myosin staining.
Next, Yogev et al looked at the effect of activity or inactivity on the expression of the transcription factor, Mef2, which they had previously shown to be needed for proper myofibril formation. As a reminder, transcription factors help to guide the transcription machinery to the right spot on the DNA to start transcribing a gene into RNA. There is a clear decrease in Mef2c (one of several Mef2 transcription factors in zebrafish) when the fish is immobilized by MS222 (below).
Continuing systematically, they now wanted to see if the decrease of Mef2c was due to breakdown of the protein, or just making less of it. What they found was that inhibiting a general protein degradation pathway did not prevent the Mef2c. This suggested that less was being made. However, they did not find any difference in the amount of Mef2c mRNAs. If the gene is still being transcribed into RNA at the same levels but there is less protein made, there is probably a decrease in translation of the RNAs into protein. As a matter of fact, this is exactly what they found.
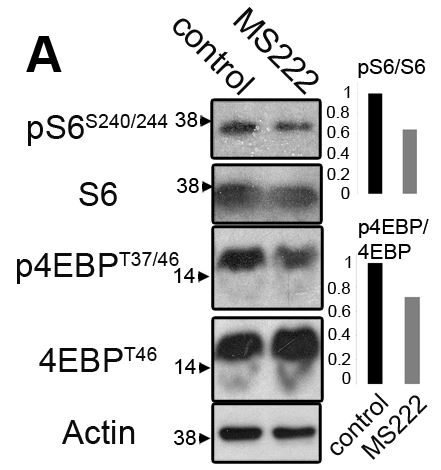
Now the question is, how does a change in muscle activity lead to downregulation of Mef2c protein synthesis? Previous work had implicated a protein kinase complex called TORC1 as being activated by muscle activity and leading to growth. Looking at targets of TORC1, they found that when eIF4EBP3L is phosphorylated by TORC1, it is unable to bind to eIF4E. This allows eIF4E to bind to the Mef2c RNA and facilitate its translation into protein. This was supported by an experiment in which they expressed eIF4EBP3L at double the normal levels and found that there was a 20% further decrease in Mef2c levels in MS222-treated fish. Conversely, when eIF4BP3L is knocked down by ~80%, MS222-induced inactivity did not significantly drop Mef2c levels.’
The figure above is slightly messy, but can be summarized in the two bar graphs to the right. In both graphs, the black bar is the control, and grey bar as the MS222-induced inactive muscle. The top graph shows the ratio of phosphorylated S6 to unphosphorylated S6. S6 part of the TORC1 complex and is activated by phosphorylation. The bottom graph is the ratio of phosphorylated to unphosphorylated eIF4EBP. Here though, the phosphorylated form is inactive.
What they concluded.
Their work supports a model in which eIF4EBP3L is the switch for activity-dependent muscle growth by the following mechanism. When there is muscle activity, TORC1 is activated, and it phosphorylates eIF4EBP3L, which then drops off of eIF4E. That allows eIF4E to bind the Mef2c RNA and help translate it into Mef2c protein. Mef2ca, as a transcription factor, can then attach to genes needed for muscle growth and initiate their production. When muscles are inactive, TORC1 is not activated, so eIF4EBP3L stays attached to eIF4E, preventing the rest of the pathway from taking place.


No comments
Be the first one to leave a comment.