Growing up in tight spaces.
The paper: Shyer, A.E. et al. (2013) Villification: how the gut gets its villi. Science 342:212-218. doi:10.1126/science.1238842
Subject areas: Developmental biology
Vocabulary:
mesenchyme – a loose connective tissue containing relatively undifferentiated embryonic cells. The cells are not very densely distributed in the extracellular matrix. Because of this, the cells can migrate in the mesenchyme quite easily. For the most part, mesenchyme is a developmental stage, and eventually differentiates into other tissue types.
smooth muscle – this is an involuntary muscle group (you cannot consciously direct smooth muscles to contract or relax) that mostly controls hollow body structures like the gut, the bladder, and blood vessels. It can also be found controlling the dilation of the iris of the eye, as well as raising the hairs on your skin when you are cold or afraid.
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biological research, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
The intestines of a chicken, or a human being for that matter, are a very complex structure specialized for its primary function. The gut is there to absorb nutrients from the food that is eaten and digested. The inner wall of the gut has to contact the digested food in order to transport the nutrients across the intestinal walls and out to the rest of the body. If the gut were completely smooth inside, it would only contact a very small amount of the food that passed through it: imagine the food as sort of a sausage: only the nutrients on the outside casing of the sausage would be absorbed, while all the nutrients inside would keep going through the intestine. How does the gut absorb more nutrients?
It increases the surface area available to contact the food, and it does so by forming many finger-like projections that cover the inner intestinal wall and reach into the digested food material to make contact with more of it. These intestinal “fingers” are called villi. And, as it turns out, they are made not by individual villi gradually poking their way inward from the intestinal lining, but by folding of the entire inner gut lining.
The mature gut has three basic layers: the innermost layer, or intestinal epithelium, that comes in contact with the food, a layer of smooth muscle that helps to move the food in one direction through the gut, and an external layer of mesothelium, that encloses and lubricates the outside of the gut so it can freely move within the abdominal cavity. Of course, the gut is not the same from top to bottom either: the esophagus and stomach are relatively smooth inside, as they are mostly there to take food in and break it down. There is some absorption of nutrients, but not a lot compared to the small intestine, which is where the bulk of nutrient absorption takes place. The lining of the small intestine has a lot of villi. Finally, the large intestine, which is more about re-absorbing water and expelling waste, is smoother than the small intestine.
We mentioned at the top that the chicken gut is similar in structure to the human, and studying development in chick embryos is relatively easy compared to mammalian experimental models, because they develop in an external egg.
What they knew.
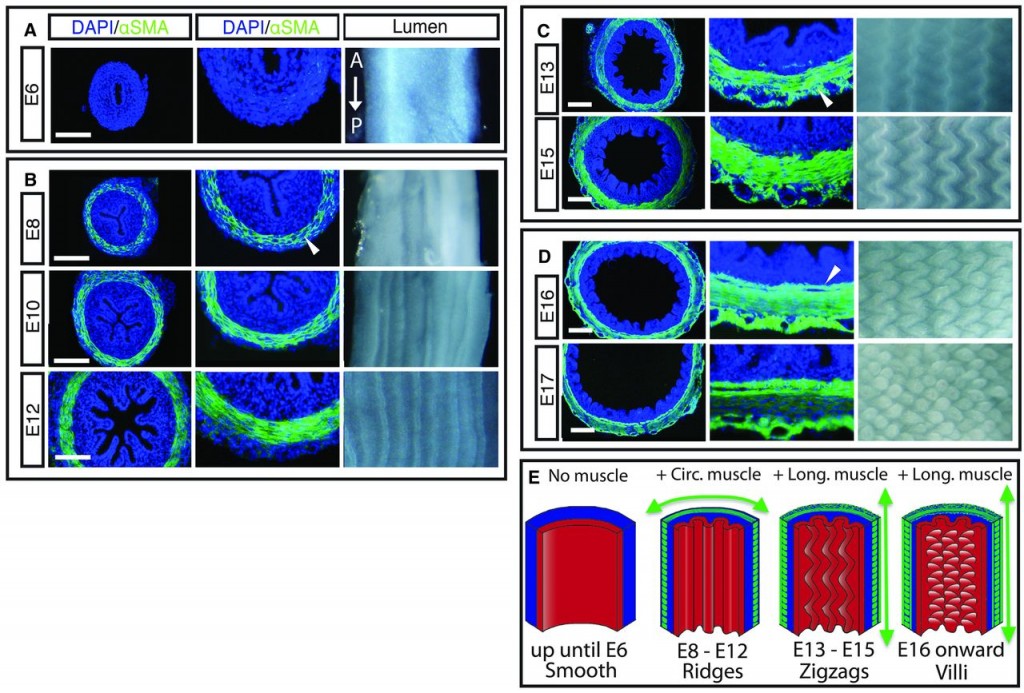
From older work, it was known that there are three stages to the development of villi, in which the intestinal epithelium folds on itself to a sort of accordion pattern, and is followed by more folding in a different direction. This appeared to involve microfilaments (also known as actin filaments) inside the epithelial cells, but little was known about the signals that caused the folding to take place. There was suspicion that it was a mechanical process more than a biochemical process, but there was little clear evidence to back that up.
What they did.
First, Shyer et al examined developing chick gut sections for both morphology (shape and structure) and for particular protein markers. This is shown below, and the results. Earlier, we mentioned a muscle layer to the intestine. In fact, it is two layers of muscle that arise at distinctly different times: first muscles that form in a circular fashion around the gut, like connected rings all along the gut. Then later, longitudinal muscles form perpendicular to the first set of muscles. The timeline is diagrammed in part E of the figure below, and what is striking is that the development of the different muscle types is coincidentally about the time of the different stages of villi formation.
Of course, at this point, they could not say if one caused the other, vice versa, or neither. Panels A-D show sections of the developing embryonic chick gut. The blue DAPI stain just shows where cells are located (it stains nuclei), while the green αSMA stain shows formation of actin microfilaments, which are part of the contraction apparatus of muscles.
There has long been a theoretical model that says that the formation of circumferential ridges of a multilayered tube could come from differential growth of the layers. If one layer grows faster than the other, but they are connected at various points, then as more material builds up between anchor points on one layer than the other, it will naturally need to fold up into a ridge to still fit in the same linear space as the slower-growing layer. The question is, could they show this? The answer is, yes.
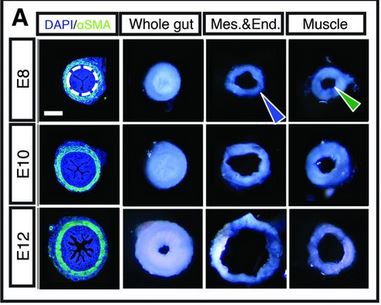 The researchers surgically separated the layers. In this figure (at right), we see cross-sections of intestine at ages E8, E10, and E12. You want to compare the “Mes.&End.” and “Muscle” pictures to the “Whole gut” picture for each age. What this shows is that the mucle layer is essentially unchanged after being separated from the mesenchymal/endothelial layer (which has the ridges). But the ridged inner layer, once it is released from the muscle, unfolds the ridges to become a much larger – in diameter – structure. Cool, right?
The researchers surgically separated the layers. In this figure (at right), we see cross-sections of intestine at ages E8, E10, and E12. You want to compare the “Mes.&End.” and “Muscle” pictures to the “Whole gut” picture for each age. What this shows is that the mucle layer is essentially unchanged after being separated from the mesenchymal/endothelial layer (which has the ridges). But the ridged inner layer, once it is released from the muscle, unfolds the ridges to become a much larger – in diameter – structure. Cool, right?
Shyer et al went further to show that if they treated E6 guts (before either ridges or muscle has formed) with a drug that blocks the differentiation into smooth muscle, then the inner layer did not form ridges, but it did grow and push outward to be a wider/more open structure than normal. That seems to say that having muscle there is needed for ridge formation, but is it because of some kind of muscle-specific communication or signal, or is it a mechanical constraint? They tested both in an experiment where they again used the drugs to block muscle differentiation, but this time, they grew the E6 guts in a silk tube of approximately the diameter the muscle would have been. Ta-da! Ridges! Also, just to check that it wasn’t something with the silk tube, they also grew the E6 guts in a much larger silk tube and the muscle-differentiation-blocking drugs, and no ridges formed.
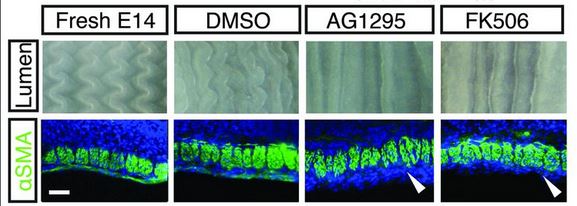
They then did a similar procedure with E14 guts, right before the longitudinal set of muscles differentiates, and the zig-zag ridges in the intestinal epithelium form. Rather than repeat all the details, the basic idea is that once again, the formation of the zig-zag ridges is dependent on muscle differentiation. Picture is below. AG1295 and FK506 are the drugs that block smooth muscle differentiation. The drugs are typically prepared in a solution of DMSO, so the DMSO alone is a control. The white arrowheads show that places where normally smooth muscle would stain green. Notice that the zig-zag is completely gone.
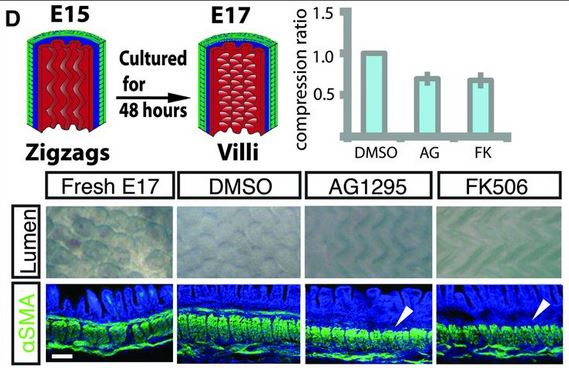
There is one more set of muscle differentiation and constraints that is needed for villi formation. And again, using AG1295 and FK506, they showed that if they blocked the formation of that last set of muscle fibers, the villi would not form, and would stay large zig-zag ridges.
Finally, the research team investigated the proliferation of the epithelial layer. Up through the formation of ridges and until zig-zags form, the actively proliferating cells are distributed throughout the layer. However, after the zig-zags have formed, the proliferation is restricted to the regions between the villi.
From the data that they gathered from these experiments, Shyer and her colleagues produced a simplified biophysical “computer model” based solely on the physical material properties of the system. One of the interesting things about this model is that it is possible to make predictions about the evolutionary conservation of these mechanisms and actually test them. Unlike the chick and humans, the African clawed frog, Xenopus, does not have villi – it has zig-zag ridges lining its intestines. It turns out that the intestinal muscles of Xenopus have only two layers, not three, and the lack of that third layer is consistent with a model of villi formation that requires it to progress from zig-zag to villi. What about the mouse, though? In the mouse, there are no ridges or zig-zags – the villi arise from a smooth epithelial layer. Actually, there is a good explanation consistent with their model. Namely, unlike in the chick, when the differentiation of the muscle layers takes eight days, all three muscle layers in the mouse gut are formed within just 48 hours, giving little time for the formation of the intermediate structures.
I chose this paper to summarize is as a reminder to myself that even as so much biological research focuses on subcellular things like proteins, DNA, mitochondria, hormones, etc, we need to constantly keep in mind that all of life exists in a physical world, with physical and mechanical properties and constraints that can also have an impact on biological processes.

No comments
Be the first one to leave a comment.