Copper and Cancer.
The paper: Brady, D.C. et al. (2014) “Copper is required for oncogenic BRAF signalling and tumorigenesis.” Nature 509:492-496. doi:10.1038/nature13180
Subject areas: molecular biology
Vocabulary:
tumorigenesis – the process by which normal cells become tumor (cancer) cells. This usually involves a number of steps: mutations in genes that control the cell cycle, part of which can be the generation of new cells by mitosis. When the mutations combine in such a way as to remove negative controls on the cell cycle or push it forward uncontrollably, tumorigenesis results.
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biology, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
If you have ever looked at the list of vitamins and minerals in a multivitamin, you may have run across names that appear to have come right off of the periodic table of the elements, and then wondered just where in the body that might actually have an effect. When most people think about copper, it is usually in terms of electrical wiring or plumbing, but it actually has a number of roles in animal physiology. The best known roles have to do with oxygen metabolism. In some animals (though not mammals), it is a part of the primary oxygen carrier in blood (similar to the role of iron in our blood) and is also found more universally in enzymes that process oxygen and oxygen compounds. This paper describes a novel new role for copper, as a critical component of the BRAF kinase signaling pathway that is overactivated in a number of different cancers, including thyroid and skin cancers, as well as some kinds of leukemia.
The BRAF kinase signaling pathway normally works like this: BRAF is a kinase, meaning it is an enzyme that adds phosphate groups onto other molecules. In this case, when BRAK is activated by something, it phosphorylates MEK1 and MEK2, thus turning them on. MEK1 and MEK2 are kinases themselves, which in turn phosphorylate and activate ERK1 and ERK2, which – you guessed it – are also kinases. Among many functions, normal stimulation of this pathway can push the cell cycle forward and lead to mitosis and more cells. However, when BRAF is mutated in such a way that it stays turned on all the time, it becomes an oncogene: it promotes cancer by constantly pushing the cell through the cell cycle to make more cells regardless of whether that is normally supposed to happen. That uncontrolled proliferation of cells forms a tumor, and depending on whether and how quickly it spreads to other parts of the body, may become a cancer.
What They Did
Their previous work showed that copper (Cu) can bind to MEK1 and increase its activity. So, they decided to see if changing the availability of copper to a cell carrying the BRAF-V600E oncogene (V600E means a mutation of BRAF where the 600th amino acid of the protein was mutated from valine to glutamate) could affect its ability to form tumors. Since MEK1 is an enzyme completely inside of cells, one of the first things the research group tested was the effect of knocking out Ctr1, the gene for a Copper transport protein that normally takes copper ions from outside of the cell and brings them inside. If you are following along with the paper and you are not familiar with signal transduction research, figure 1 is probably overwhelming and confusing. Brady et al have embryonic mouse cells that either have the normal complement of Ctr1 genes (Ctr1 +/+) or have had both Ctr1 genes knocked out (Ctr1 -/-). Into some of these cells, they introduced the oncogene BRAF-V600E.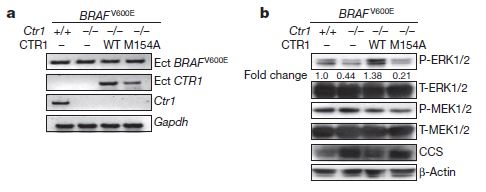 Figure 1a is RT-PCR, which means that they are looking at expression of the genes in the cells, essentially double-checking that the cells are producing what they are supposed to (or not). So if you look at the third row, labeled Ctr1, only the far left column has a signal, since it contains RNA extracted from cells that are Ctr1 +/+. All the other columns show no signal, as they all come from cells that are Ctr1-knockouts (-/-). Looking across the top row, all extracts show expression of BRAF-V600E as expected, since that gene was introduced into all the cells. The second row corresponds to the re-introduction of Ctr1 genes into two of the samples. Finally, Gapdh levels are a control for the experiment. It is a common metabolic gene that should be in all cells at roughly equal levels, so this shows that the technical aspects of the experiment are probably OK.
Figure 1a is RT-PCR, which means that they are looking at expression of the genes in the cells, essentially double-checking that the cells are producing what they are supposed to (or not). So if you look at the third row, labeled Ctr1, only the far left column has a signal, since it contains RNA extracted from cells that are Ctr1 +/+. All the other columns show no signal, as they all come from cells that are Ctr1-knockouts (-/-). Looking across the top row, all extracts show expression of BRAF-V600E as expected, since that gene was introduced into all the cells. The second row corresponds to the re-introduction of Ctr1 genes into two of the samples. Finally, Gapdh levels are a control for the experiment. It is a common metabolic gene that should be in all cells at roughly equal levels, so this shows that the technical aspects of the experiment are probably OK.
That’s all fine and dandy, but it doesn’t actually tell us anything about the biology of the system, it just says that the setup of the experiment is basically valid. Figure 1b gets more to the point. These figures are protein levels, and more importantly, they also show both phosphorylated versions of the proteins (prefixed by “P-“) and total amounts of the proteins (prefixed by “T-“). This means we can see if the genes that were introduced have an effect on phosphorylation/activation of key parts of the MAPK signaling cascade. ERK and MEK have already been described, CCS is a copper chaperone protein that should be needed in higher amounts when copper is scarce, and the actin is a structural protein that should be the same in all cells, so it acts as a control for the amount of total protein loaded into each lane for analysis. This and the other parts of figure 1 not shown here demonstrate, using a variety of genetically constructed and inserted components of the BRAF/MAP kinase cascade, that copper binds specifically to MEK1, and that when it does so, MEK1 becomes more active and thus promotes greater activation of the MAPK pathway and subsequently, increases tumor formation.
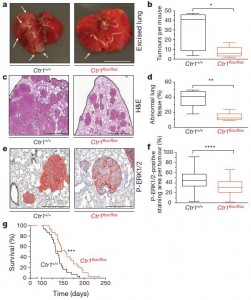 Importantly, knocking out Ctr1 (decreasing copper in cells) in a mouse engineered with the cancer-causing BRAF-V600E mutation can lower the incidence of tumors. The figure to the right shows examples of lung sections from BRAF-cancer mice that have either normal Ctr1 (left panels in a, c, and e) or knocked out Ctr1 (right panels). The graphs more clearly show the significant differences caused by knocking out copper transport into the cell, and in the bottom one, even demonstrate that the survival of the animals is significantly increased.
Importantly, knocking out Ctr1 (decreasing copper in cells) in a mouse engineered with the cancer-causing BRAF-V600E mutation can lower the incidence of tumors. The figure to the right shows examples of lung sections from BRAF-cancer mice that have either normal Ctr1 (left panels in a, c, and e) or knocked out Ctr1 (right panels). The graphs more clearly show the significant differences caused by knocking out copper transport into the cell, and in the bottom one, even demonstrate that the survival of the animals is significantly increased.
Although the genetic knockout results are exciting, from a therapeutic standpoint they are not very helpful because introducing genes as a medical intervention is currently severely limited by available technology. One method is to use the drug vemurafenib, which inhibits BRAF. However, cancers can sometimes develop a resistance to it, so its effectiveness can be limited. So, the researchers looked into ways to control the amount of copper that gets into cells by using drugs that chelate (binds up) copper. Of the tested compounds, TTM (tetrathiomolybdate) was the most effective, and this was even better when the mice were fed diets deficient in copper. 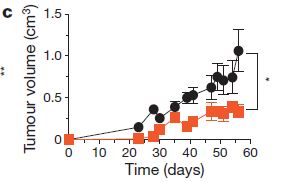 The figure to the left shows that the tumor volume is lower in the TTM-treated mice (orange) compared to control mice (black). Unfortunately, so far it appears that tumor growth restarts when treatment is stopped, but it may be the first step in developing a more effective treatment for BRAF-V600E-involved cancers.
The figure to the left shows that the tumor volume is lower in the TTM-treated mice (orange) compared to control mice (black). Unfortunately, so far it appears that tumor growth restarts when treatment is stopped, but it may be the first step in developing a more effective treatment for BRAF-V600E-involved cancers.

No comments
Be the first one to leave a comment.