Wound healing, regeneration, and Lin28
The paper: Shyh-Chang, N. et al. (2013) Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155(4):778-792. doi:10.1016/j.cell.2013.09.059
Subject areas: Cell Biology, Developmental Biology
Vocabulary:
oncogene – a gene that can cause cells to form a tumor. The oncogene is a mutated form of a normal gene, so that it somehow becomes overactive, either because too many copies are being made, or it is hyperactive, or it always stays on, instead of being able to be switched on and off. The normal genes that can become oncogenes when mutated are referred to as protooncogenes.
—–
This article is a summary of a recent primary research paper intended for high school teachers to add to their general knowledge of current biology, or to supplement their lessons by showing students the kinds of projects that current biological research addresses.
—–
When the average student is asked about RNA, the usual answer is that RNA is an intermediate step for turning the information stored in the DNA sequence into a protein that performs some function for the cell or organism. That particular type of RNA is called messenger RNA (mRNA). However, there are several other types of RNA that exist and function solely as RNA, and are never translated into a protein.
One of these other types is the micro RNA, or miRNA. As the name implies, they are very small, generally less than 25 nucleotides. By way of comparison, mRNAs can be thousands of nucleotides long. The primary function of most miRNAs is to downregulate gene expression. Though the mechanism of the downregulation can vary, one of the key characteristics of miRNA function is that it can be highly specific because it relies on the complementary base-pairing between its own sequence and the sequence of the mRNA it targets. You recall that base-pairing is the basis of DNA’s double-stranded nature, and means that adenines will form hydrogen bonds with thymine (or uracil for RNA) and guanines will form hydrogen bonds with cytosine. Basically, when the miRNA sequence is exactly or very close to complementary to a target, it will form a little double-stranded section with it. For example, if the miRNA has AGUAA as part of its sequence, it will zip together with a target that has UCAUU in its sequence. This can lead to a variety of downregulating mechanisms including cleavage of the target RNA, destabilization of the RNA, and inhibition of translation.
There is a class of miRNA called the let-7 miRNAs. These are evolutionarily highly conserved, with great sequence similarity all the way from mammals to the tiny worm Caenorhabditis elegans. There are twelve mammalian let-7 miRNAs, expressed at specific times and tissue types during development. They are thought to be involved in a variety of developmental processes including control of differentiation and proliferation.
The subject of today’s paper, Lin28, is a key regulator of let-7-family miRNA. It represses the formation of let-7 by binding onto the precursor RNA and preventing it from being processed into a fully functional miRNA. It has also been implicated as an oncogene – when it is overactive, it can lead to cancer. It has been found in leukemias and cancers of the breast, colon, lung, liver, and other organs.
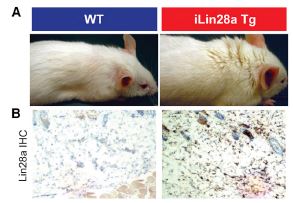
Shyh-Chang and colleagues created an inducibly-overexpressing transgenic Lin28 mouse. That means that when the mouse is given the otherwise harmless antibiotic doxycycline, Lin28a is overexpressed. However, it is a “leaky” overexpresser, so that means that even when not induced, it is producing slightly higher levels of Lin28a than wild-type mice. One of the first things that the researchers noticed about Lin28a mice, even un-induced, was that they appeared to have much thicker coats of fur and thicker skin. As it happens, there is also an increase in Lin28a expression in the epidermis (dark staining).
Unexpectedly though, the density of hair follicles was no different between wild-type and Lin28a mice, nor were the hair bulbs any larger (i.e. individual hairs were not significantly thicker). What could be causing the thicker fur? Could it have something to do with hair growth? They knew that normal hair follicles go through a cycle of growth and resting phases, and just after birth, these growth (anagen) and resting (telogen) phases are consistently timed in normal wild-type mice. Interestingly, when they carefully examined the anagen/telogen cycle in the Lin28a mice, they found that telogen was greatly shortened and anagen periods were significantly longer than wild-type.
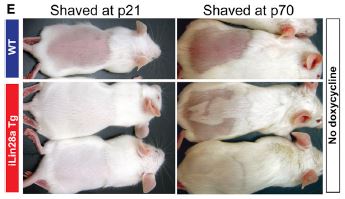
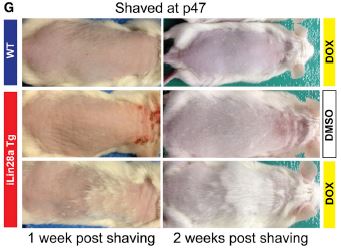
To investigate the mechanism of this difference, the researchers shaved the backs of both Lin28a mice and their wild-type littermates during what should be a telogen phase (above). You can see that there is variability in the Lin28a mice, but even un-induced, the hair regrowth is faster than wild-type. The left panels are mice one week after being shaved at 21 days old, and the right panels are mice three weeks after being shaved at 70 days old. This suggests that the Lin28a mice are either not in telogen or are more quickly shifting into anagen. Further study showed that Lin28a mice were not actually in telogen at p21 or p70, but are at p47. So, in the panel below, we see the result of shaving the mice when both wild-type and Lin28a mice are known to be in telogen. Upon inducing Lin28a with doxycycline, it is clear that the hair follicles are forced into anagen and are starting to grow hair.
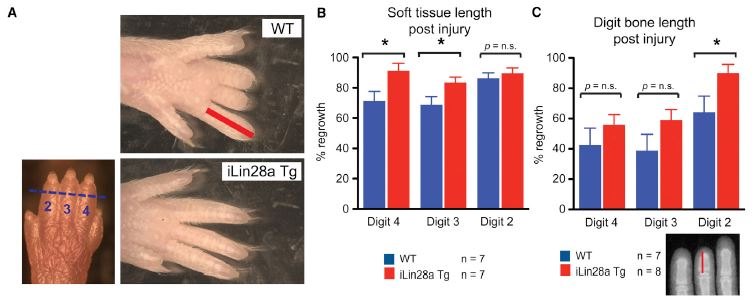
During normal development, Lin28a is found in multiple tissues at various times, so other differences were anticipated. Lin28a is expressed in early limb formation but then drops sharply and is very low at birth. The investigators tested regenerative capabilities, and found significant increases in regeneration after amputation of the distal segment of toes 2, 3, and 4. Interestingly, soft tissue regeneration was only statistically significant for digits 3 and 4, but bone regeneration was only significant for digit 2; however, this could simply be due to the sample size. There was also over 200 times as much Lin28a expression in the digits of newborn Lin28a mice than in wild-type. Although the increased regeneration was promising in the newborns, it turns out that in adults, there was no significant difference in regeneration. Lin28a may play a role in regeneration, but clearly is not sufficient by itself to promote regeneration of digits.
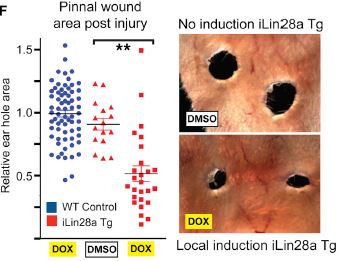
Examining another model of wound healing, the researchers looked for regeneration after pinnal punches (2 mm holes in the earlobes). The data below show healing of pinnal holes ten days after injury, with and without extra induction of Lin28a expression by doxycycline. Looking at the molecular mechanism of this, it was assumed that since the main function of Lin28a is to repress let-7 miRNA production, this might also be how the regeneration works. In fact, when a similar experiment was done on mice that overexpress let-7, wound closure and repair of the pinna was inhibited. Surprisingly though, when a different method for repressing let-7 was used, neither pinnal tissue repair nor hair regrowth was observed.
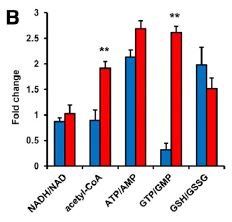
What else is Lin28a doing? It was already known that Lin28a is involved in glucose metabolism, so they looked at the levels of various metabolic indicators. In blue is the increase in wild-type injured tissue over uninjured tissue, and in red is the increase in Lin28a injured tissue over uninjured tissue. The Lin28a-expressing mice have much larger increases in their acetyl-CoA level and the ration of GTP to GMP, as well as an increase in ATP/AMP ratio, all indicating increased energy production. How much of this is due to repression of let-7 miRNA? Again using an alternate let-7 inhibitor, the research team found that while some of the same bioenergetic increases were seen, the changes in the Krebs cycle/oxidative phosphorylation were not observed. So, independently of let-7, Lin28a promotes oxidative enzyme expression in regenerating tissues.
These experiments provide new insight into the processes of wound repair and regeneration, demonstrating a coordinated need for regulating genes involved in differentiation and proliferation as well as those involved in providing more energy to an area that requires more than usual to accomplish the repairs and remodeling.

No comments
Be the first one to leave a comment.